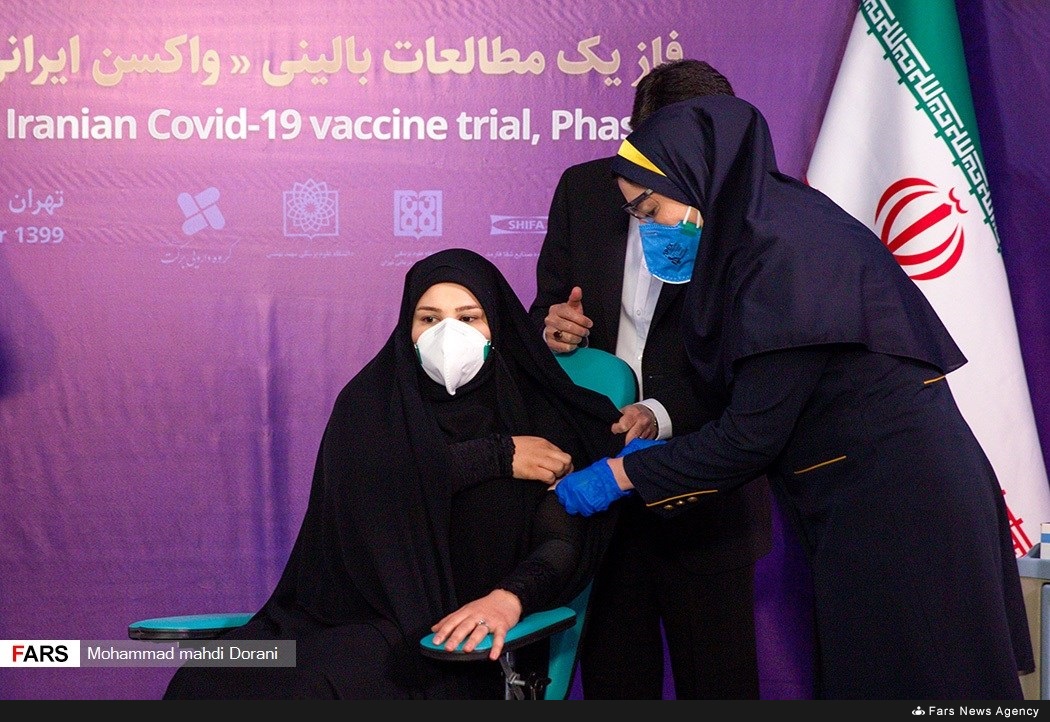
Iran Begins Human Trial of Its COVID-19 Vaccine
30 December 2020
TEHRAN (Dispatches) -- Iran launched Tuesday the first phase of a clinical trial of a coronavirus vaccine developed in the Islamic Republic in the face of U.S. sanctions which have hampered the country’s access to the medical gear for the pandemic.
"The first vaccine against the coronavirus, developed by Iranian researchers, has been unveiled by injecting three people,” the Islamic Republic of Iran Broadcasting (IRIB) reported.
The novel coronavirus has killed nearly 55,000 out of more than 1.2 million people infected in Iran, according to health ministry data.
While food and medicine are allegedly exempt from the U.S. sanctions, international banks tend to refuse transactions involving Iran.
President Hassan Rouhani on Saturday said Washington had demanded that Tehran pay for the drugs through U.S. banks and said he feared the United States would seize the money.
IRIB on Tuesday broadcast images at the trial launch showing two men and one woman receiving injections in the presence of the health minister and the Iranian vice-president in charge of science and technology.
The vaccine, produced by Shifa Pharmed, part of a state-owned pharmaceutical conglomerate, is the first in the country to reach human trials.
The report said the three volunteers were the daughter of the president of EIKO and two high-ranking officials of the conglomerate.
"I am happy that the scientific process went ahead in a proper way,” said Tayebeh Mokhber, daughter of the Barekat Pharmaceutical Group chairman, who was the first to get jabbed. "I hope the conclusion will be health for our people.”
The treatment, dubbed Coviran, is a so-called inactivated vaccine, meaning that it is made of a coronavirus that has been weakened or killed by chemicals, similar to how polio immunizations are made. Leading Western vaccines, such as the shot made by Pfizer and its German partner BioNTech, use newer, less-proven technology to target the coronavirus’ spike protein using RNA.
The vaccine will be administered to "56 volunteers” in two doses two weeks apart, according to Iribnews, quoting a person in charge of developing the drug.
The result would be announced 28 days after the second injection, it said.
"From the next two or three weeks, we are ready to produce 1.5 million doses of the coronavirus vaccine in Iran every month,” Barekat Pharmaceutical Group chairman Muhammad Mokhber said.
Another Iranian vaccine, developed at the country’s Razi Institute, "will receive authorization” for the start of human trials "in the very near future,” said Health Minister Saeed Namaki.
Rouhani has said Iran is cooperating with a "foreign country” to produce the second vaccine
expected to run in tests in human volunteers in February.
Tehran said last month that eight of the vaccines it is formulating locally against COVID-19 had made their way onto the World Health Organization (WHO)’s list of 48 promising candidate vaccines.
"We will prove in the near future that the homegrown vaccine (for COVID-19) is better than many vaccines being produced (elsewhere) in the world,” Namaki said during a virtual press conference on Sunday.
An adviser to Namaki denounced last week the promotion of the coronavirus vaccines produced by U.S. companies Pfizer and Moderna by some sections of the Iranian media.
In parallel with efforts at developing home-made vaccines, government officials have said the county has plans to pre-purchase foreign brands that pass safety and efficiency tests.
Namaki early this month said Iran had "pre-purchased” about 16.8 million vaccine doses via Covax, the mechanism for the equitable distribution of vaccines established by the UN World Health Organization.
Source: http://kayhan.ir/en/news/86034/iran-begins-human-trial-of-its-covid19-vaccine